Naoh Hcl Balanced Equation
Chemical equation Balanced chemical equation implications of a balanced chemical equation types of chemical reactions. Unit-I a.

How To Write The Net Ionic Equation For Hcl Naoh Nacl H2o Youtube
1 a 0b 2 c O.
. In the synthesis reactions the final product is the combination of reactants involved in the chemical reaction such as follows. For each method state the chemicals you will need and write a balanced chemical equation for the reaction. A Al b O 2 c Al 2 O 3.
FeCl 3 NaOH NaCl FeOH 3. It shows the number of. Two other examples of this reaction are given below and.
For example in the reaction. Fe Au Co Br C O N F. Balanced chemical equation AgNO3 aq HCl aq AgCl s HNO3 aq The reaction type is.
Combination decomposition displacement double. Mg HCl MgCl 2. 0800 mol of water is equivalent to 144g of water.
From the balanced chemical equation 1 mole NaOH reacts with 1 mole of HCl So 803 10-3 mole NaOH reacted with 803 10-3 moles HCl The amount of HCl that was added to the chalk in excess was 803 10-3 mol Step 2. Determine the amount of calcium carbonate in chalk. How many grams of calcium bromide are in 500 mL of a 025 M calcium.
Once you know how many of each type. First I wrote the balanced chemical equation. The permanganate oxidant is reduced usually to MnIV or MnII.
To balance NaOH HCl NaCl H2O youll need to be sure to count all of atoms on each side of the chemical equation. Balanced chemical equation for the reaction if one of the compounds formed is calcium chloride. A salt is formed when an acid is neutralised by a base.
NaOH HCl NaCl H2O Eg. The balancing chemical equations calculator highlights you whether your equation is balanced or not. The Kp of AgI is 83 x 10-17.
22 WHAT DO ALL ACIDS AND ALL BASES HAVE IN. Label each compound reactant or product in the equation. The balanced equation will appear above.
Refer to the table of activity coefficients at various ionic strengths as needed. HKC3H4O4s NaOH. P 2 O 5 H 2 O H 3 PO 4.
C 6 H 6 HNO 3 heat. In general a neutralisation reaction. The balanced chemical equation for the reaction between PCl.
Label Each Compound With a Variable. Titration methods using phenolphthalein. C 6 H 6 Cl 2 heat FeCl 3 catalyst C 6 H 5 Cl HCl Chlorobenzene.
Use uppercase for the first character in the element and lowercase for the second character. 2 2KOH H2SO4 K2SO4 2H2O Chapter 10 Acids Bases and Salts. Cl or Br Nitration.
HClaq NaOHaq NaClaq H 2 Ol heat Since theses are dilute solutions and are mostly water assume that the densities of the solutions and the specific heat capacities of the solutions are approximately 10 gml and 418. The method of chemical titration is employed to find unknown concentrations of acids or bases by finding their neutralization point. From the balanced chemical equation I concluded that 0800 mol of water must have been produced too.
HCl NaOH NaCl H 2 O. P ΔH ΔQ ΔV. Solution for Write the products of the reaction between the acid KHP and the base NaOH and balance the reaction.
How many moles of O₂ are required to form 500 moles of H₂O. Application of Neutralization Reaction 1. 4 5 HCl.
Zn HCl ZnCl 2 H 2. I know that 0800 mol of NaOH and 0800 mol of HCl was consumed. 0a 2 b 3 c.
If 345 moles of HCl are produced how many moles of water reacted. Label each compound reactant or product in the equation with a variable to represent the unknown coefficients. FeSO 4 NaOH Na 2 SO 4 FeOH 2.
I used q mcΔT and I wrote. In order to practice different methods of balancing chemical equations the following unbalanced equations can be worked on. Find the concentration of I in 0010 M AGNO saturated with AgI.
And water is given below. Models a chemical reaction using the formulae. Include activity coefficients in the solubility-product expression.
The mole ratio between H₂ and H₂O is 2 mol H₂2 mol H₂O. 5 4 H. To find the point where the neutralization happens we use a pH indicator or pH meter.
Balance the equation HCl NaOH NaCl H2O using the algebraic method. The reactions were performed with 200 nM enzyme in 600 µl of 100 mM KH 2 PO 4-NaOH buffer pH 80 and terminated at 6 12 18 or 24 h for quantifying total PET monomers released at each time point. This is the balanced form of the given chemical equation.
Typical Equation Electrophile E Halogenation. 47 Solution to Quick check 4 1. HCl NaOH NaCL H 2 O.
By making considerable change in enthalpy equation we get. Q 144g x 418 x 277-251. NaOHaq HClaq NaClaq H 2 Ol The reaction between an acid and a base to give a salt and water is known as a neutralisation reaction.
It shows the number of units of each substance involved. Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations. 2H₂g O₂g 2H₂Og The mole ratio between O₂ and H₂O is 1 mol O₂2 mol H₂O.
For Sulphuric acid H2SO4 the valency is 2 as you get 2 H ions when you dissolve it in water. These equations are not balanced. Create an equation for each element Al O where each term represents the number of atoms of the element in each reactant or product.
A 0690 mol b 0863 mol c 276 mol d 345 mol e 431 mol. Lets say a molecule HNO3 is having a normality of 05. T he balanced chemical equation representing the neutralization of hydrochloric acid with sodium hydroxide is.
HCl NaOH on the basis of their reaction with. Solution Chapter 10 Acids Bases and Salts 47. P 7 41 11.
However an online Chemical Equation Balancer Calculator will provide you the balanced equation equilibrium constant with chemical name and formula of all reactants and product of a chemical equation. Create a System of Equations. For HCl Hydrochloric acid valency is one because when you dissolve it in the water you get one H ion.
For NaOH valency is one as it gives one OH- ion when it dissolves in water.

Naoh Hcl Nacl H2o Hoh Chemical Equation Balancer

How To Balance Naoh Hcl Nacl H2o Sodium Hydroxide Plus Hydrochloric Acid Youtube

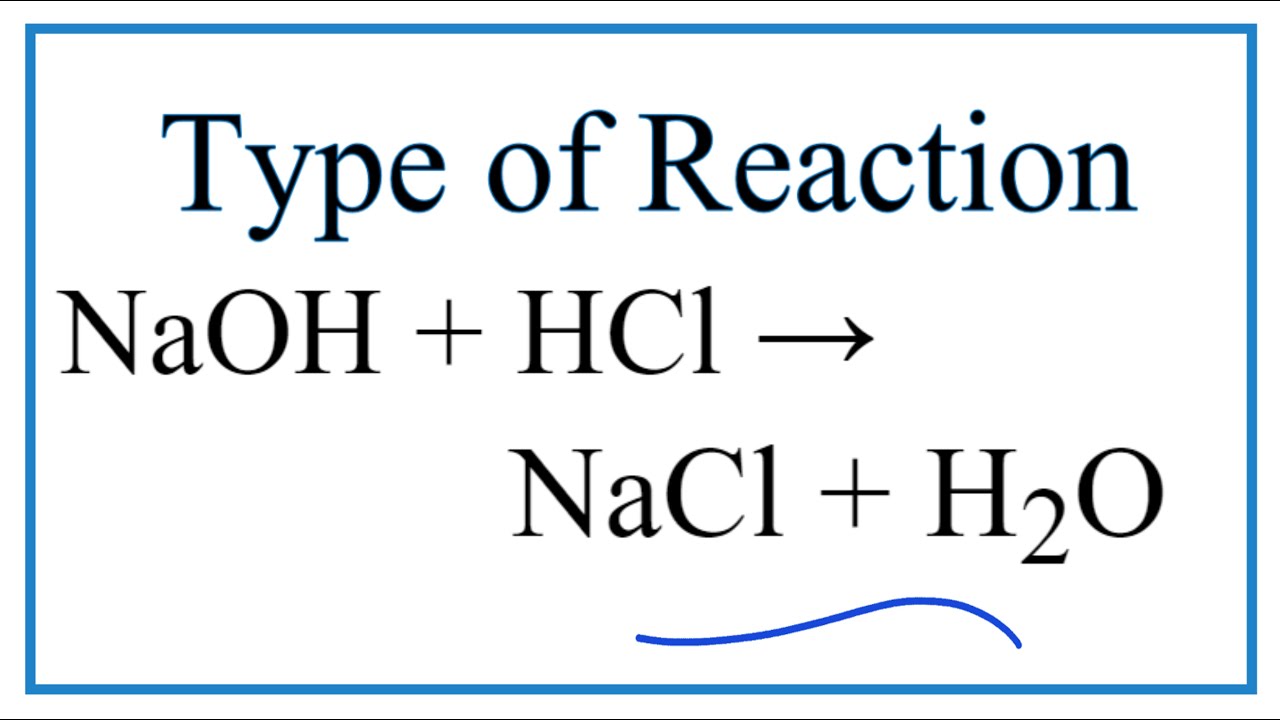
Type Of Reaction For Naoh Hcl Nacl H2o Youtube

How To Balance Naoh Hcl Nacl H2o Sodium Hydroxide Plus Hydrochloric Acid Youtube
Comments
Post a Comment